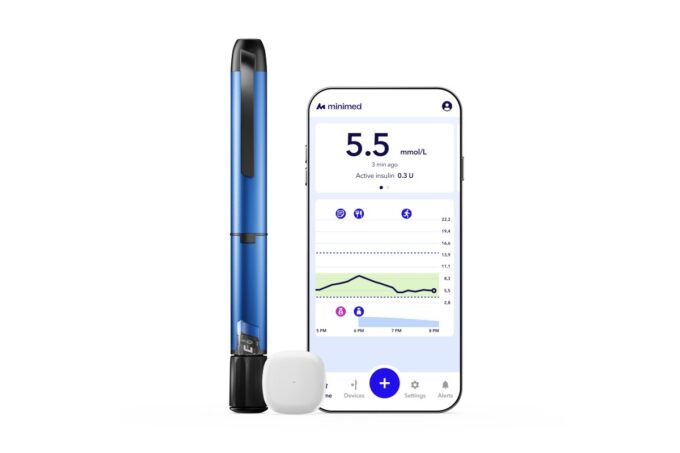
Medtronic Diabetes has received U.S. FDA 510(k) clearance for the MiniMed Go™ Smart Multiple Daily Injection system. The system brings together the InPen™ smart insulin pen and the Instinct glucose sensor from Abbott through the MiniMed Go™ app.
MiniMed Go is the first smart MDI system that automatically combines insulin dosing and glucose data in a single app. It delivers real time insights like missed dose alerts a dose calculator and clear guidance when a dose is missed or miscalculated. The system also connects with CareLink software to support easier collaboration with healthcare providers.
The system is cleared for people with insulin requiring type 1 and type 2 diabetes aged seven years and above. Children aged two to six years can use it under adult supervision. Compatibility with the Simplera sensor is currently under FDA review.
MiniMed Go is designed to reduce the daily burden of managing multiple injections. Real world data from earlier Medtronic smart MDI systems shows that timely responses to alerts can significantly improve time in range and overall glucose control.
The commercial launch of MiniMed Go in the U.S. is expected to begin this spring.
















Leave a comment