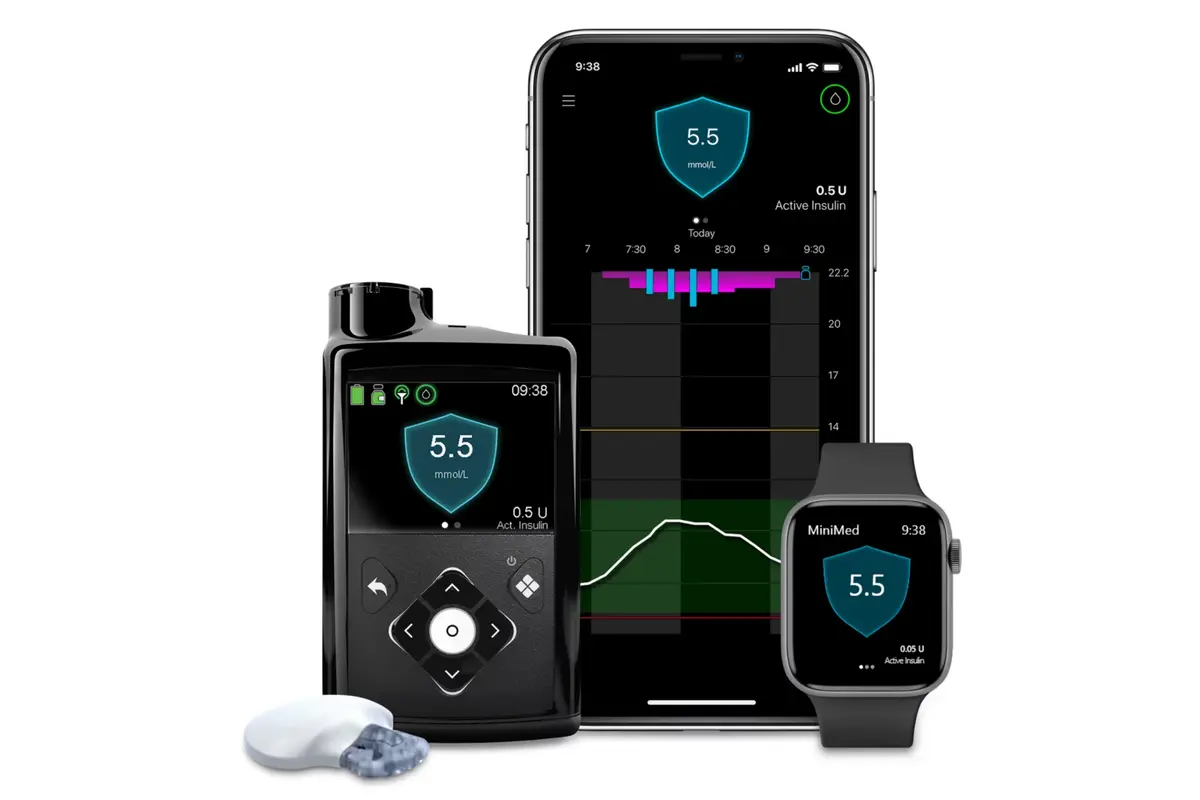
Medtronic announced three US milestones that expand access and flexibility for people with type 1 and insulin requiring type 2 diabetes. These include Medicare access for the MiniMed 780G system with the Instinct sensor made by Abbott, FDA clearance for use with ultra rapid acting insulins and FDA clearance for use in insulin requiring type 2 diabetes.
Medicare and Medicare Advantage beneficiaries can now access the MiniMed 780G system with the Instinct sensor which offers up to 15 days of wear. This adds to existing sensor options and allows more Medicare users to adopt automated insulin delivery that adjusts insulin every five minutes to help manage glucose levels.
The U.S. Food and Drug Administration also cleared the MiniMed 780G system for use with ultra rapid acting insulins Fiasp and Lyumjev. These insulins act faster around mealtimes and when paired with Meal Detection technology can help manage missed or delayed meal doses.
In addition, the system was cleared for insulin requiring type 2 diabetes when used with the Instinct sensor. The MiniMed 780G system automatically adjusts insulin delivery and supports people managing complex daily diabetes routines.
















Leave a comment