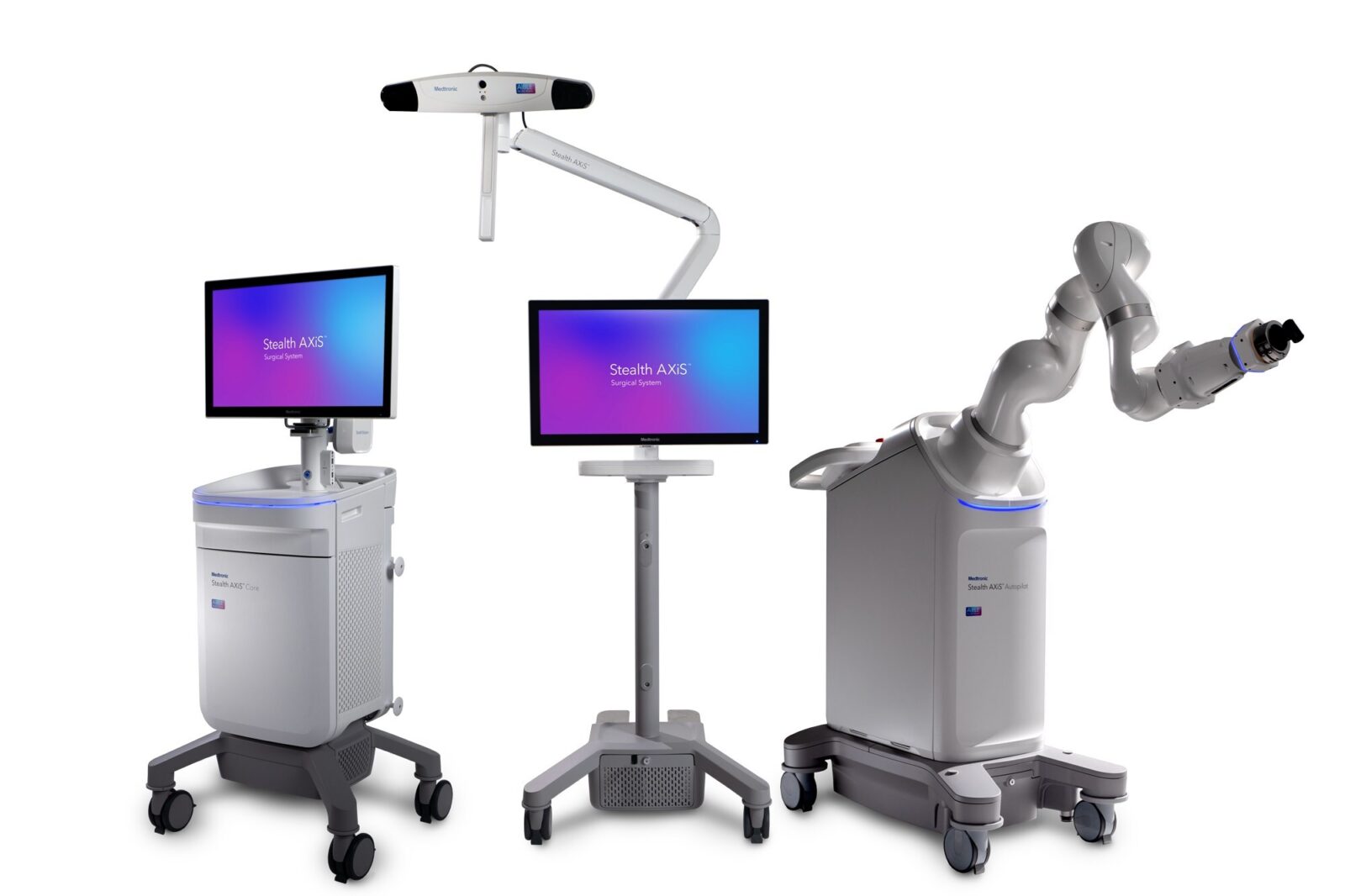
Medtronic has received U.S. FDA clearance for the Stealth AXiS surgical system, an integrated platform that combines planning, navigation and robotics for spine surgery.
The system is cleared for spine procedures in the United States and is designed to support future cranial and ENT applications pending additional clearance. It integrates surgical planning, real time navigation and modular robotics into a single platform for use in hospitals and ambulatory surgery centers.
A key feature is LiveAlign segmental tracking, which allows surgeons to visualize spinal motion and alignment in real time during procedures without repeated imaging. The platform is part of Medtronic’s AiBLE smart ecosystem, enabling connected workflows and data exchange across the surgical process.
The system builds on Medtronic’s experience in surgical navigation and robotics and is designed to support personalized surgical planning and more consistent execution in complex spine procedures.














Leave a comment